Calculate the average atomic mass. 6) Copper used in electric wires comes in two flavors (isotopes): 63Cu and 65Cu. 63Cu has an atomic mass of 62.9298 amu and an abundance of 69.09%. The other isotope, 65Cu, has an abundance of 30.91%. The average atomic mass between these two isotopes is 63.546 amu. Calculate the actual atomic mass of 65Cu. How can you find the average atomic mass of an element, if you're given the isotopic abundances and the mass of each isotope? In nature, there are three types of magnesium: Mg-24 has a mass of 23.985 amu and accounts for 78.70% of all magnesium Mg-25 has a mass of 24.985 amu and accounts for 10.13% of all magnesium.

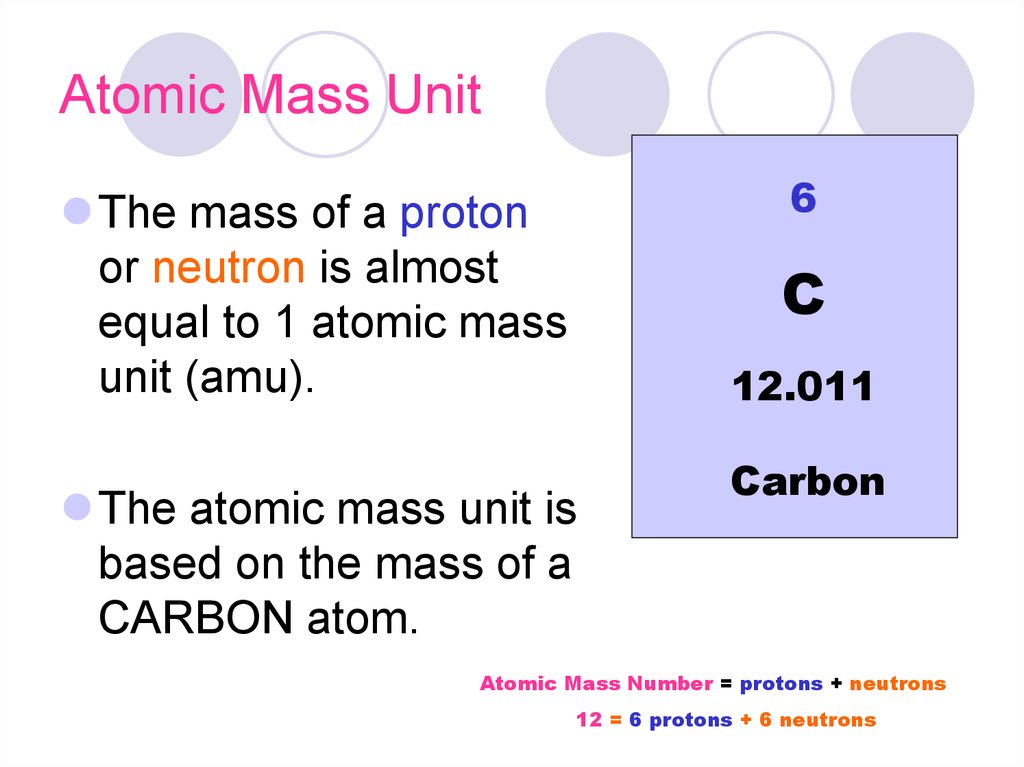
The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D). The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope. (Atomic mass is also referred to as atomic weight, but the term 'mass' is more accurate.)
How To Calculate The Average Atomic Mass
The term average atomic mass is a average, and so is calculated differently from a normal average. Explain how this type of average is calculated. Average Atomic Mass Masses of Atoms A scale designed for atoms gives their small atomic masses in atomic mass units (amu) An atom of 12C was assigned an exact mass of 12.00 amu Relative masses of all other atoms was determined by comparing each to the mass of 12C An atom twice as heavy has a mass of 24.00 amu. Os x quartz.

For instance, it can be determined experimentally that neon consists of three isotopes: neon-20 (with 10 protons and 10 neutrons in its nucleus) with a mass of 19.992 amu and an abundance of 90.48%, neon-21 (with 10 protons and 11 neutrons) with a mass of 20.994 amu and an abundance of 0.27%, and neon-22 (with 10 protons and 12 neutrons) with a mass of 21.991 amu and an abundance of 9.25%. The average atomic mass of neon is thus:
| 0.9048 | × | 19.992 amu | = | 18.09 amu |
| 0.0027 | × | 20.994 amu | = | 0.057 amu |
| 0.0925 | × | 21.991 amu | = | 2.03 amu |
| 20.18 amu |
The atomic mass is useful in chemistry when it is paired with the mole concept: the atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of an element. Thus, since the atomic mass of iron is 55.847 amu, one mole of iron atoms would weigh 55.847 grams. The same concept can be extended to ionic compounds and molecules. One formula unit of sodium chloride (NaCl) would weigh 58.44 amu (22.98977 amu for Na + 35.453 amu for Cl), so a mole of sodium chloride would weigh 58.44 grams. One molecule of water (H2O) would weigh 18.02 amu (2×1.00797 amu for H + 15.9994 amu for O), and a mole of water molecules would weigh 18.02 grams.
The original periodic table of the elements published by Dimitri Mendeleev in 1869 arranged the elements that were known at the time in order of increasing atomic weight, since this was prior to the discovery of the nucleus and the interior structure of the atom. The modern periodic table is arranged in order of increasing atomic number instead.
What is the average atomic mass of silicon?
Parallels 7 download. Given the following data, calculate the average atomic mass of silicon.
isotope = Si-28
amu =27.9769
abundance(%) =92.18
isotope = Si-29
amu =28.9765
abundance(%) =4.71
isotope =Si-30
amu =29.9738
abundance(%) =3.12
Parallels 7 download. Given the following data, calculate the average atomic mass of silicon.
isotope = Si-28
amu =27.9769
abundance(%) =92.18
isotope = Si-29
amu =28.9765
abundance(%) =4.71
isotope =Si-30
amu =29.9738
abundance(%) =3.12
2.8: The Average Mass Of An Element’s Atoms - Chemistry ..
1 Answer
Multiply the amu by the percentage of occurrence to arrive at an average atomic mass of
Explanation:
We take the amu of each isotope, multiply it by the percentage of occurrence, and end up with a weighted average:
This simplifies to:

Checking 'the internet' to verify our answer, I found 28.0855 u ± 0.0003 u, so the answer calculated for our question is pretty close.
Related questions