- Atomic Number Of Nine
- Atomic Number Of Nitrate
- Atomic Number Of Nickel
- Atomic Number Of Nitrogen 14
- Atomic Number Of Nitride
[NiCl4]2− is paramagnetic, while [Ni(CO)4] is diamagnetic, though both are tetrahedral. Why? (Atomic number of Ni = 28)
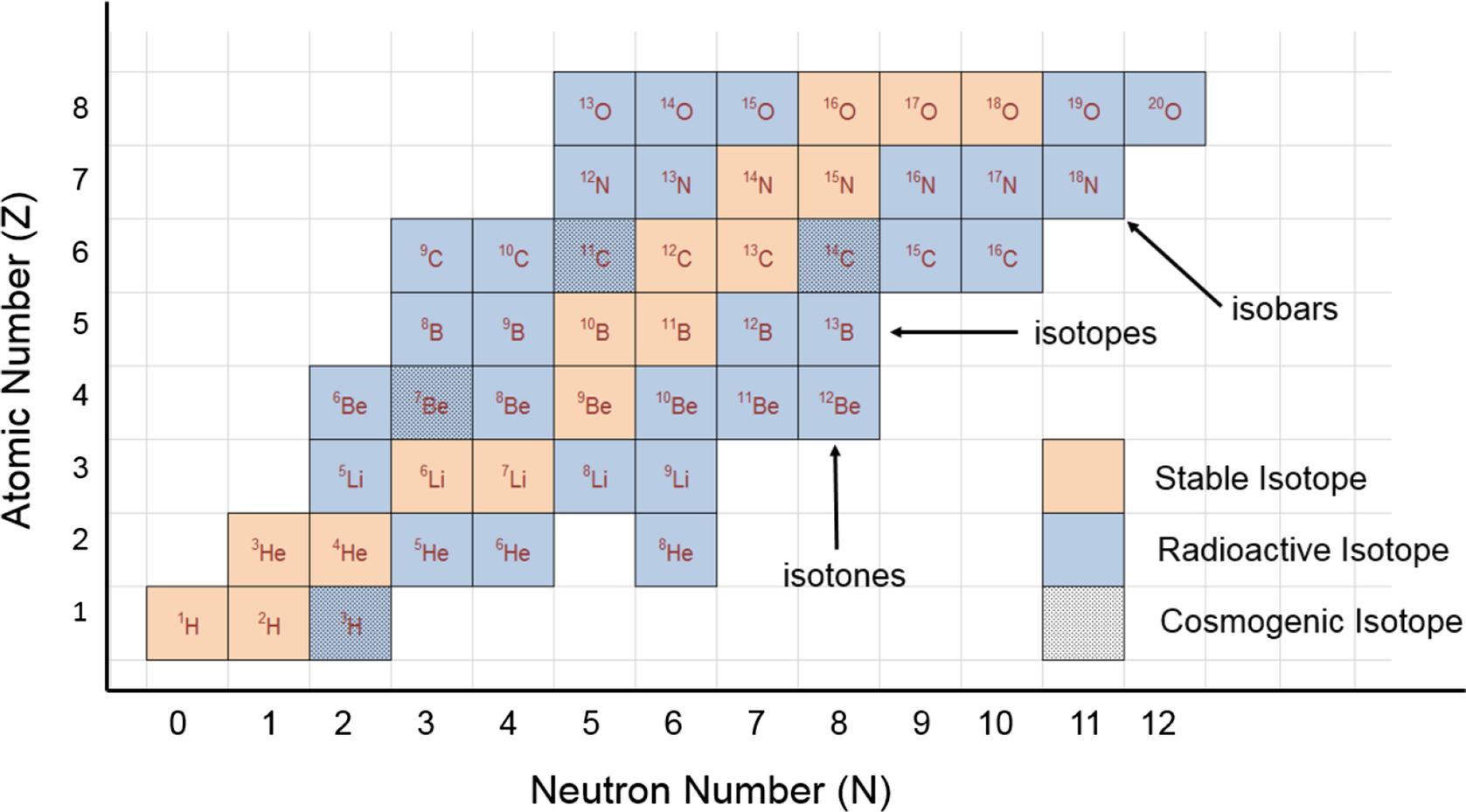
Atomic number = Number of protons. For example, in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11. Atomic Number Orbital Energy Levels. The most common way of showing the arrangement of electrons in an atom is to draw diagrams like those shown in the diagram. Find Atomic number of over 118 known elements like atomic number of Hydrogen, Helium, Carbon, Sodium, Nitrogen, Oxygen, Iron, Silicon, Aluminum, Copper, Silver, Gold, Platinum, Phosphorus, Magnesium and other elements Atomic number of Nickel (Ni) is 28 view full reference table.
The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that.
Why is [NiCl4]2– paramagnetic but [Ni(CO)4] is diamagnetic ? (At. nos. : Cr = 24, Co = 27, Ni = 28)
Solution 1
In [NiCl4]2−, Ni is in the +2 state. Cl− is a ligand which is a weak field ligand which does not cause pairing of unpaired 3d electrons. Hence, it is paramagnetic. In [Ni(CO)4], Ni has 0 oxidation state. CO is a strong field ligand, which causes pairing of unpaired 3d electrons. No unpaired electrons are present in this case. So, it is diamagnetic.

Solution 2
In [NiCl4]2−, the oxidation state of Ni is +2. Chloride is a weak field ligand and does not cause pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, two unpaired electrons are present in the valence d-orbitals of Ni which impart paramagnetic character to the complex. On the other hand, carbonyl is a strong field ligand and causes pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, no unpaired electrons are present and hence, the complex is diamagnetic.
Solution 3
[NiCl4]2− and [Ni(CO)4] both are tetrahedral. But their magnetic characters are different. This is due to difference in the nature of ligands.
Ni+2 = [Ar] 4s03d8
Atomic Number Of Nine
Ni+2 has 2 unpaired electrons hence, this complex is paramagnetic.
In Ni(CO)4, Ni is in the zero oxidation state i.e., it has a configuration of 3d84s2.
Atomic Number Of Nitrate
[Ni(CO)4]
Ni = [Ar] 4s2 3d8
But CO is a strong field ligand. Therefore, it causes the pairing of unpaired 3d electrons. Also, it causes the 4s electrons to shift to the 3d orbital, thereby giving rise to sp3 hybridization. Since, no unpaired electrons are present in this case, [Ni(CO)4] is diamagnetic.
APPEARS IN
Atomic Number Of Nickel
Atomic Number Of Nitrogen 14
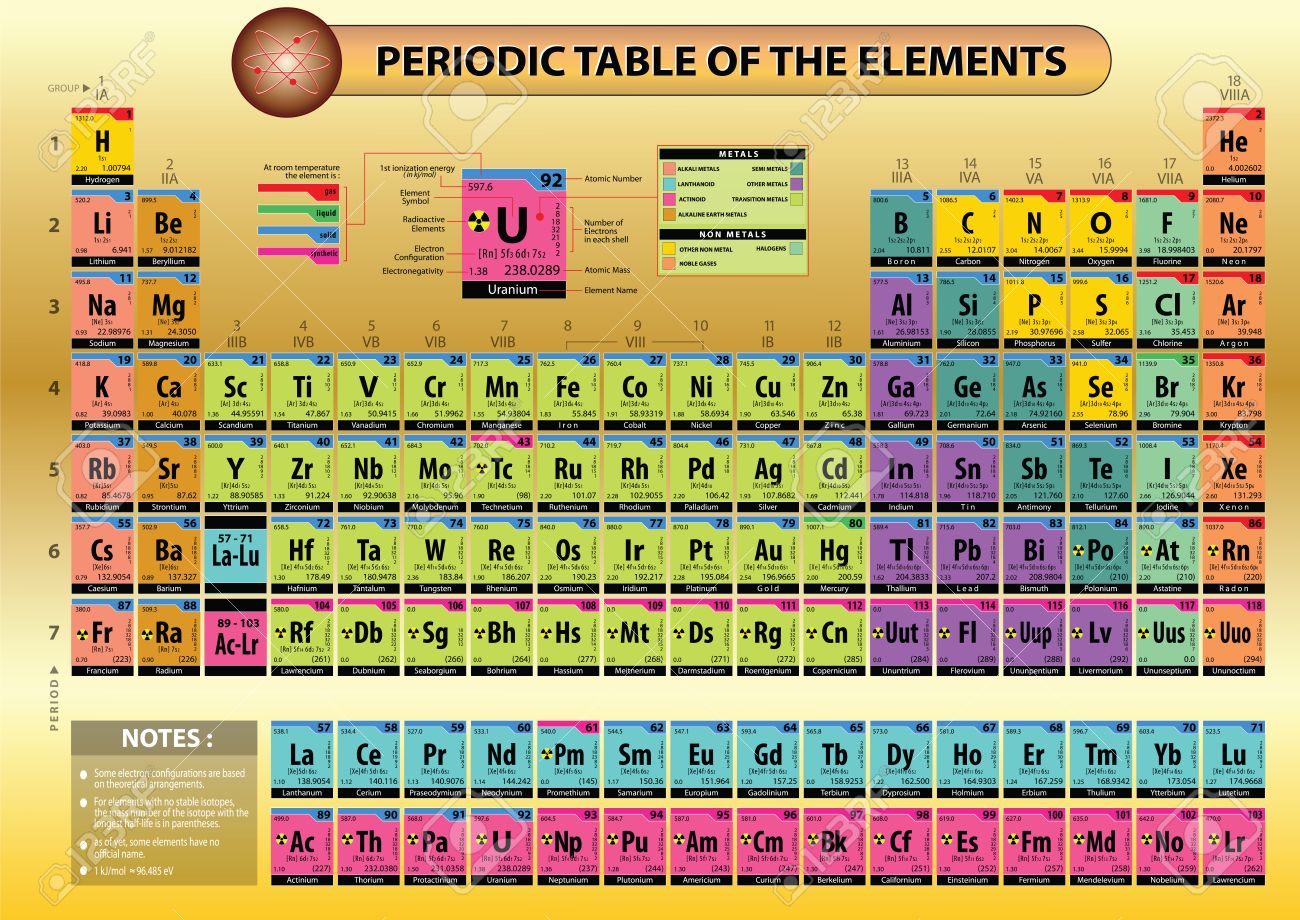
In the modern periodic table, the elements are listed in order of increasing atomic number. The atomic number is the number of protons in the nucleus of an atom. The number of protons define the identity of an element (i.e., an element with 6 protons is a carbon atom, no matter how many neutrons may be present). The number of protons determines how many electrons surround the nucleus, and it is the arrangement of these electrons that determines most of the chemical behavior of an element.
In a periodic table arranged in order of increasing atomic number, elements having similar chemical properties naturally line up in the same column (group). For instance, all of the elements in Group 1A are relatively soft metals, react violently with water, and form 1+ charges; all of the elements in Group 8A are unreactive, monatomic gases at room temperature, etc. In other words, there is a periodic repetition of the properties of the chemical elements with increasing mass.
Atomic Number Of Nitride
In the original periodic table published by Dimitri Mendeleev in 1869, the elements were arranged according to increasing atomic mass— at that time, the nucleus had not yet been discovered, and there was no understanding at all of the interior structure of the atom, so atomic mass was the only guide to use. Once the structure of the nucleus was understood, it became clear that it was the atomic number that governed the properties of the elements.
